- All Categories
- Bibliography
- Chromogenic monopack
- Chromolytic multilayer
- Color separation
- Double-coated / bi-pack
- Edge Codes and Identification
- Hand coloring
- Other
- Printing / dye-transfer
- Printing / pigment process
- Screen processes
- Spatial synthesis (multiple lenses, beam splitter)
- Stencil coloring (pochoir, Pathécolor)
- Temporal synthesis (rotary filters)
- Theory
- Tinting
- Toning
-
![]() "Du Hauron invented his Chromographoscope in 1874. It could be used either as a camera or an additive viewer." Source: Coote, Jack H. (1993): The Illustrated History of Colour Photography. Surbiton, Surrey: Fountain Press.
"Du Hauron invented his Chromographoscope in 1874. It could be used either as a camera or an additive viewer." Source: Coote, Jack H. (1993): The Illustrated History of Colour Photography. Surbiton, Surrey: Fountain Press.
- An early three-color print by Louis Ducros Du Hauron, 1877.
2 Images
-
![]() „Film orthochromatique (film négatif Pathé Standard)“ (orthochromatic stock). Source: Didiée, L. (1926): Le Film vierge Pathé. Manuel de développement et de tirage. Paris: Pathé.
„Film orthochromatique (film négatif Pathé Standard)“ (orthochromatic stock). Source: Didiée, L. (1926): Le Film vierge Pathé. Manuel de développement et de tirage. Paris: Pathé.
- „Film ordinaire (non orthochromatique)“ (Normal, non-orthochromatic stock). Source: Didiée, L. (1926): Le Film vierge Pathé. Manuel de développement et de tirage. Paris: Pathé.
2 Images
-
![]() Pénichon, Sylvie (2013): Twentieth Century Colour Photographs. The Complete Guide to Processes, Identification & Preservation. London, Los Angeles: Thames & Hudson.
Pénichon, Sylvie (2013): Twentieth Century Colour Photographs. The Complete Guide to Processes, Identification & Preservation. London, Los Angeles: Thames & Hudson.
1 Image
-
![]() Source: Coe, Brian (1978): Colour Photography. The First Hundred Years 1840-1940. London: Ash & Grant.
Source: Coe, Brian (1978): Colour Photography. The First Hundred Years 1840-1940. London: Ash & Grant.
- Source: Lavédrine, Bertrand (2009): Photographs of the Past. Process and Preservation. Los Angeles: Getty Publications.
- Source: Coote, Jack H. (1993): The Illustrated History of Colour Photography. Surbiton, Surrey: Fountain Press.
- Source: Pénichon, Sylvie (2013): Twentieth Century Colour Photographs. The Complete Guide to Processes, Identification & Preservation. London, Los Angeles: Thames & Hudson, p. 19.
- Source: Pénichon, Sylvie (2013): Twentieth Century Colour Photographs. The Complete Guide to Processes, Identification & Preservation. London, Los Angeles: Thames & Hudson, p. 235.
5 Images
Coloring of individual frames by the use of very fine brushes. The process was previously applied to lantern slides. Any water based translucent dye was suited for the process, most often the coloring was done with acid dyes.
-
![]() Loïe Fuller (FRA 1905, Anonymous). Credit: BFI National Archive. Photographs of the hand colored nitrate print by Olivia Kristina Stutz, ERC Advanced Grant FilmColors.
Loïe Fuller (FRA 1905, Anonymous). Credit: BFI National Archive. Photographs of the hand colored nitrate print by Olivia Kristina Stutz, ERC Advanced Grant FilmColors.
- Métamorphoses du papillon (FRA 1904, Gaston Velle). Credit: Library of Congress. Photograph of the nitrate prints by Barbara Flueckiger.
- Credit: Turconi Collection by courtesy of George Eastman Museum, Moving Image Collection. Film: Zara.
- Credit: Paolo Cherchi Usai. Source: Cherchi Usai, Paolo (2000): Silent Cinema. London: BFI.
- Source: Coe, Brian (1981): The History of Movie Photography. Westfield, N.J.: Eastview Editions.
250 Images in 18 Galleries
-
![]() Credit: Illustration by Sarah Steinbacher, Multimedia & E-Learning-Services, University of Zurich. Source: Ede, François (1994): Jour de fête ou la couleur retrouvée. Cahiers du Cinéma: Paris.
Credit: Illustration by Sarah Steinbacher, Multimedia & E-Learning-Services, University of Zurich. Source: Ede, François (1994): Jour de fête ou la couleur retrouvée. Cahiers du Cinéma: Paris.
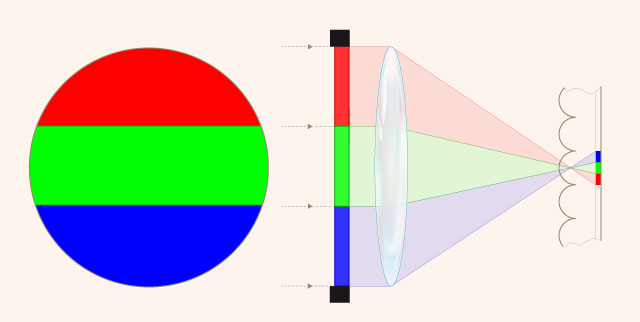
- Principle of capturing and projecting lenticular film. Credit: Joakim Reuteler and Rudolf Gschwind, Digital Humanities Lab, University of Basel, Switzerland. Illustration by Sarah Steinbacher, Multimedia & E-Learning-Services, University of Zurich.
- Principle of capturing and projecting lenticular film. Credit: Joakim Reuteler and Rudolf Gschwind, Digital Humanities Lab, University of Basel, Switzerland. Illustration by Sarah Steinbacher, Multimedia & E-Learning-Services, University of Zurich.
- Principle of capturing and projecting lenticular film. Credit: Joakim Reuteler and Rudolf Gschwind, Digital Humanities Lab, University of Basel, Switzerland. Illustration by Sarah Steinbacher, Multimedia & E-Learning-Services, University of Zurich.
4 Images
In contrast to tinting, toning is not the simple immersion of a film into a dye bath but involves a chemical reaction converting the silver image. In this reaction the neutral silver image in the emulsion of the positive film is replaced by one consisting of colored metal compounds. These were usually iron ferrocyanide (Prussian Blue) for blue, copper ferrocyanide for red/brown, silver sulfide for sepia or rarely uranium ferrocyanide for reddish brown. Toning had been used in still photography before. But since film was projected on the screen it required translucent toning compounds.
-
![]() Hongarije (FRA 1926, Anonymous). Credit: EYE Film Museum. Photographs of the tinted, toned and stencil colored nitrate print by Olivia Kristina Stutz, ERC Advanced Grant FilmColors.
Hongarije (FRA 1926, Anonymous). Credit: EYE Film Museum. Photographs of the tinted, toned and stencil colored nitrate print by Olivia Kristina Stutz, ERC Advanced Grant FilmColors.
- Virages sur films à support teinté Pathé, Film teinté lavande (virage bleu) lavender tinted stock with blue toning, the same image from a different copy of the book, combination of tungsten backlight with daylight toplight. Photograph by Barbara Flueckiger. Source: Didiée, L. (1926): Le Film vierge Pathé. Manuel de développement et de tirage. Paris: Pathé. [quote id='16']
- Photomicrograph, 25x. Credit: Norbert Wey, Institute of Pathology, University of Zurich.
- Photomicrograph, 50x. Credit: Norbert Wey, Institute of Pathology, University of Zurich.
- Photomicrograph, 100x. Credit: Norbert Wey, Institute of Pathology, University of Zurich.
- Toning samples from the Tinting and Toning Workshop by Ulrich Ruedel, Seminar "Materiality of Film" by Barbara Flueckiger and Bregt Lameris, Department of Film Studies, University of Zurich, in collaboration with Lichtspiel / Kinemathek Bern, David Landolf and Brigitte Paulowitz.
- Toning samples from the Tinting and Toning Workshop by Ulrich Ruedel, Seminar "Materiality of Film" by Barbara Flueckiger and Bregt Lameris, Department of Film Studies, University of Zurich, in collaboration with Lichtspiel / Kinemathek Bern, David Landolf and Brigitte Paulowitz.
1549 Images in 62 Galleries
For tinting, the positive print is immersed into a variety of dye baths, scene by scene. To this end, the print has to be cut into the corresponding fragments and reassembled after the dyeing process. The dye homogeneously attaches over the entire image’s gelatin including the perforation area. Usually synthetic dyes were dissolved in a weak acid solution to form a chemical bond with the gelatin.
-
![]() Salomé (USA 1922, Charles Bryant). Credit: George Eastman Museum. Photographs of the tinted, toned and Handschiegl nitrate print by Barbara Flueckiger.
Salomé (USA 1922, Charles Bryant). Credit: George Eastman Museum. Photographs of the tinted, toned and Handschiegl nitrate print by Barbara Flueckiger.
- Photograph by Barbara Flueckiger. Source: Agfa Kine-Handbuch. Berlin: Actien-Gesellschaft für Anilin-Fabrikation. (around 1927, estimated).
- See Mazzanti (2009: 71). Credit: Cineteca di Bologna. Film: Lyda Borelli in Malombra (Italy, 1917).
- As coloured for South America, see Mazzanti (2009: 71). Credit: Cineteca di Bologna.
- As coloured for the domestic Italian market, see Mazzanti (2009: 71). Credit: Cineteca di Bologna.
- Photomicrograph, 25x. Credit: Norbert Wey, Institute of Pathology, University of Zurich.
- Photomicrograph, 50x. Credit: Norbert Wey, Institute of Pathology, University of Zurich.
- Photomicrograph, 50x. Credit: Norbert Wey, Institute of Pathology, University of Zurich.
- Credit: Lichtspiel / Kinemathek Bern. Film: Dagli Appennini alle Ande (ITA 1916, Umberto Paradisi). Photograph by Barbara Flueckiger.
- Credit: Lichtspiel / Kinemathek Bern. Film: Dagli Appennini alle Ande (ITA 1916, Umberto Paradisi). Photograph by Barbara Flueckiger.
- Credit: Lichtspiel / Kinemathek Bern. Film: Dagli Appennini alle Ande (ITA 1916, Umberto Paradisi). Photograph by Barbara Flueckiger.
- Credit: Lichtspiel / Kinemathek Bern. Film: Dagli Appennini alle Ande (ITA 1916, Umberto Paradisi). Photograph by Barbara Flueckiger.
- Credit: Lichtspiel / Kinemathek Bern. Film: Dagli Appennini alle Ande (ITA 1916, Umberto Paradisi). Photograph by Barbara Flueckiger.
- Credit: Lichtspiel / Kinemathek Bern. Film: Dagli Appennini alle Ande (ITA 1916, Umberto Paradisi). Photograph by Barbara Flueckiger.
- Credit: Lichtspiel / Kinemathek Bern. Film: Gaumont Woche.
- Credit: Lichtspiel / Kinemathek Bern. Film: Gaumont Woche.
- Credit: Lichtspiel / Kinemathek Bern. Film: Gaumont Woche.
- Credit: Lichtspiel / Kinemathek Bern. Film: Gaumont Woche.
- Credit: Lichtspiel / Kinemathek Bern. Film: Selznick News.
4492 Images in 111 Galleries
-
![]() Photomicrograph (20x) of a Joly screen. Credit: Courtesy of George Eastman House, International Museum of Photography and Film.
Photomicrograph (20x) of a Joly screen. Credit: Courtesy of George Eastman House, International Museum of Photography and Film.
- Credit: Courtesy of George Eastman House, International Museum of Photography and Film.
- Source: Coe, Brian (1978): Colour Photography. The First Hundred Years 1840-1940. London: Ash & Grant.
- Credit: Gawain Weaver, Photograph Conservator, Gawain Weaver Art Conservation, San Anselmo, CA.
- Source: Pénichon, Sylvie (2013): Twentieth Century Colour Photographs. The Complete Guide to Processes, Identification & Preservation. London, Los Angeles: Thames & Hudson, p. 69.
- Source: Pénichon, Sylvie (2013): Twentieth Century Colour Photographs. The Complete Guide to Processes, Identification & Preservation. London, Los Angeles: Thames & Hudson, p. 22.
- Source: Pénichon, Sylvie (2013): Twentieth Century Colour Photographs. The Complete Guide to Processes, Identification & Preservation. London, Los Angeles: Thames & Hudson, p. 23.
7 Images
-
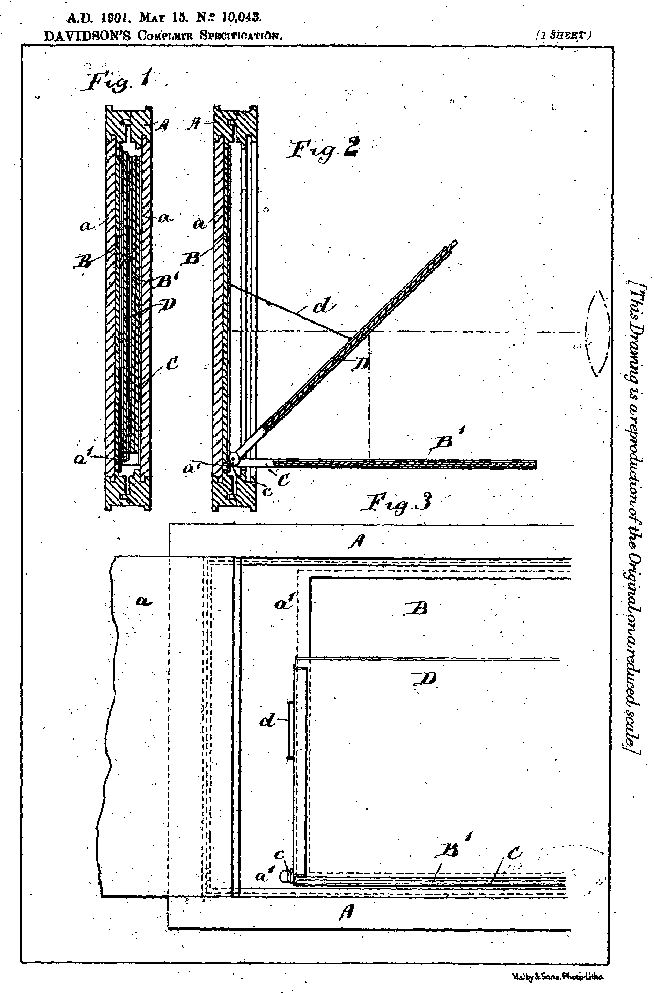
![]() Source: D.R.P. 98,799, Dec. 17, 1897
Source: D.R.P. 98,799, Dec. 17, 1897
- Credit: Illustration by Sarah Steinbacher, Multimedia & E-Learning-Services, University of Zurich. Source: D.R.P. 98,799, Dec. 17, 1897
2 Images
-
![]() Source: Hübl, Arthur Freiherr von (1904): Die Dreifarbenphotographie mit besonderer Berücksichtigung des Dreifarbendruckes und der photographischen Pigmentbilder in natürlichen Farben. Halle a. S.: Druck und Verlang von Wilhelm Knapp. Photograph by Martin Weiss, ERC Advanced Grant FilmColors.
Source: Hübl, Arthur Freiherr von (1904): Die Dreifarbenphotographie mit besonderer Berücksichtigung des Dreifarbendruckes und der photographischen Pigmentbilder in natürlichen Farben. Halle a. S.: Druck und Verlang von Wilhelm Knapp. Photograph by Martin Weiss, ERC Advanced Grant FilmColors.
- Source: Hübl, Arthur Freiherr von (1904): Die Dreifarbenphotographie mit besonderer Berücksichtigung des Dreifarbendruckes und der photographischen Pigmentbilder in natürlichen Farben. Halle a. S.: Druck und Verlang von Wilhelm Knapp. Photograph by Martin Weiss, ERC Advanced Grant FilmColors.
- Source: Hübl, Arthur Freiherr von (1904): Die Dreifarbenphotographie mit besonderer Berücksichtigung des Dreifarbendruckes und der photographischen Pigmentbilder in natürlichen Farben. Halle a. S.: Druck und Verlang von Wilhelm Knapp. Photograph by Martin Weiss, ERC Advanced Grant FilmColors.
- Source: Hübl, Arthur Freiherr von (1904): Die Dreifarbenphotographie mit besonderer Berücksichtigung des Dreifarbendruckes und der photographischen Pigmentbilder in natürlichen Farben. Halle a. S.: Druck und Verlang von Wilhelm Knapp. Photograph by Martin Weiss, ERC Advanced Grant FilmColors.
- Source: Hübl, Arthur Freiherr von (1904): Die Dreifarbenphotographie mit besonderer Berücksichtigung des Dreifarbendruckes und der photographischen Pigmentbilder in natürlichen Farben. Halle a. S.: Druck und Verlang von Wilhelm Knapp. Photograph by Martin Weiss, ERC Advanced Grant FilmColors.
- Source: Hübl, Arthur Freiherr von (1904): Die Dreifarbenphotographie mit besonderer Berücksichtigung des Dreifarbendruckes und der photographischen Pigmentbilder in natürlichen Farben. Halle a. S.: Druck und Verlang von Wilhelm Knapp. Photograph by Martin Weiss, ERC Advanced Grant FilmColors.
- Source: Hübl, Arthur Freiherr von (1904): Die Dreifarbenphotographie mit besonderer Berücksichtigung des Dreifarbendruckes und der photographischen Pigmentbilder in natürlichen Farben. Halle a. S.: Druck und Verlang von Wilhelm Knapp. Photograph by Martin Weiss, ERC Advanced Grant FilmColors.
- Source: Hübl, Arthur Freiherr von (1904): Die Dreifarbenphotographie mit besonderer Berücksichtigung des Dreifarbendruckes und der photographischen Pigmentbilder in natürlichen Farben. Halle a. S.: Druck und Verlang von Wilhelm Knapp. Photograph by Martin Weiss, ERC Advanced Grant FilmColors.
- Source: Hübl, Arthur Freiherr von (1904): Three-Colour Photography. Three-Colour Printing and the Production of Photographic Pigment Pictures in Natural Colours. London: W.A. Penrose.
- Source: Hübl, Arthur Freiherr von (1904): Die Dreifarbenphotographie mit besonderer Berücksichtigung des Dreifarbendruckes und der photographischen Pigmentbilder in natürlichen Farben. Halle a. S.: Druck und Verlang von Wilhelm Knapp. Photograph by Martin Weiss, ERC Advanced Grant FilmColors.
11 Images
-
![]() [U-Boot]. Credit: Deutsches Filminstitut DIF. Photograph of the chromolithographic nitrate print by Barbara Flueckiger.
[U-Boot]. Credit: Deutsches Filminstitut DIF. Photograph of the chromolithographic nitrate print by Barbara Flueckiger.
- Box Der Film fürs Heimkino. Credit: Deutsches Filminstitut DIF. iPhone photo by Barbara Flueckiger.
- Box "Film for Home Cinema". Credit: Deutsches Filminstitut DIF. iPhone photo by Barbara Flueckiger.
- Box "Attention! Celluloid Film". Credit: Deutsches Filminstitut DIF. iPhone photo by Barbara Flueckiger.
- Boxes and loops. Credit: Deutsches Filminstitut DIF. iPhone photo by Barbara Flueckiger.
- Box and loops. Credit: Deutsches Filminstitut DIF. iPhone photo by Barbara Flueckiger.
- Film can for Chromolithographic Loop. Credit: Deutsches Filminstitut DIF. iPhone photo by Noemi Daugaard, SNSF Filmcolors
- Film can for Chromolithographic Loop. Credit: Deutsches Filminstitut DIF. iPhone photo by Noemi Daugaard, SNSF Filmcolors
119 Images in 3 Galleries
“In 1898 William Friese-Greene, a professional portrait photographer by trade, demonstrated in London ‘the first process of true natural-color cinematography.’ His program consisted of ‘a series of animated natural-color pictures,’ and although this demonstration aroused considerable interest at the time, Friese-Greene was unable to exploit this system on a profitable basis. Undaunted, he eventually developed a total of four different color methods.”
-
![]() Credit: Courtesy of BFI National Archive. Photograph by Barbara Flueckiger. Film: Kino the Girl of Colour (GB 1920, William Friese-Greene, Claude Friese-Greene).
Credit: Courtesy of BFI National Archive. Photograph by Barbara Flueckiger. Film: Kino the Girl of Colour (GB 1920, William Friese-Greene, Claude Friese-Greene).
- Credit: Cinémathèque française, conservatoire des techniques, Paris.
- Credit: Paolo Cherchi Usai. Source: Cherchi Usai, Paolo (2000): Silent Cinema. London: BFI.
112 Images in 2 Galleries
-
![]() „Their projector […] used three lenses and a special filter
wheel which enabled every frame of the film to be projected three times in succession through the appropriate filter as it passed through the triple frame projector aperture. Although it eventually led to the Kinemacolor two colour process, the Lee and Turner patent was not successful“. Source: Coote, Jack H. (1993): The Illustrated History of Colour Photography. Surbiton, Surrey: Fountain Press.
„Their projector […] used three lenses and a special filter
wheel which enabled every frame of the film to be projected three times in succession through the appropriate filter as it passed through the triple frame projector aperture. Although it eventually led to the Kinemacolor two colour process, the Lee and Turner patent was not successful“. Source: Coote, Jack H. (1993): The Illustrated History of Colour Photography. Surbiton, Surrey: Fountain Press.
- "The First Color Moving Pictures", from the YouTube channel of the National Media Museum. [quote id="1"]
- Lee Turner projector in the Media History Museum in Bradford.
- „In 1899 Lee and Turner obtained a patent for a three colour process of cinematography requiring a single lens camera […] making a recurring sequence of red green and blue exposures through a rotating filter cum shutter“. Source: Coote, Jack H. (1993): The Illustrated History of Colour Photography. Surbiton, Surrey: Fountain Press.
- „In 1899 Lee and Turner obtained a patent for a three colour process of cinematography requiring a single lens camera […] making a recurring sequence of red green and blue exposures through a rotating filter cum shutter“. Source: Coote, Jack H. (1993): The Illustrated History of Colour Photography. Surbiton, Surrey: Fountain Press.
- „Their projector […] used three lenses and a special filter wheel which enabled every frame of the film to be projected three times in succession through the appropriate filter as it passed through the triple frame projector aperture. Although it eventually led to the Kinemacolor two colour process, the Lee and Turner patent was not successful“. Source: Coote, Jack H. (1993): The Illustrated History of Colour Photography. Surbiton, Surrey: Fountain Press.
- National Media Museum, UK. Lee and Turner Colour Projector, 1902.
- Source: Klein, Adrian Bernhard (Cornwell-Clyne) (1940): Colour Cinematography. Boston: American Photographic Pub. Co.
- Illustration of the process from National Media Museum's short documentary.
- 38 mm positive of the Lee Turner film showing the three b/w images for the three color records. Credit: Brian Pritchard.
16 Images in 1 Gallery
Photographs of unidentified color film technologies. Several different principles and times. Feel free to contact us if you can help identifying them!
-
![]() Unidentified Processes from the Kodak Film Samples Collection and the Cinematography Collection.
Credit: National Science and Media Museum Bradford.
Photographs by Barbara Flueckiger in collaboration with Noemi Daugaard, SNSF Film Colors.
Unidentified Processes from the Kodak Film Samples Collection and the Cinematography Collection.
Credit: National Science and Media Museum Bradford.
Photographs by Barbara Flueckiger in collaboration with Noemi Daugaard, SNSF Film Colors.
- Unidentified Processes from the Kodak Film Samples Collection and the Cinematography Collection. Credit: National Science and Media Museum Bradford. Photographs by Barbara Flueckiger in collaboration with Noemi Daugaard, SNSF Film Colors.
- Unidentified Processes from the Kodak Film Samples Collection and the Cinematography Collection. Credit: National Science and Media Museum Bradford. Photographs by Barbara Flueckiger in collaboration with Noemi Daugaard, SNSF Film Colors.
298 Images in 5 Galleries
-
![]() Source: Coe, Brian (1978): Colour Photography. The First Hundred Years 1840-1940. London: Ash & Grant, p. 54.
Source: Coe, Brian (1978): Colour Photography. The First Hundred Years 1840-1940. London: Ash & Grant, p. 54.
- Source: Pénichon, Sylvie (2013): Twentieth Century Colour Photographs. The Complete Guide to Processes, Identification & Preservation. London, Los Angeles: Thames & Hudson, p. 34.
- Source: Pénichon, Sylvie (2013): Twentieth Century Colour Photographs. The Complete Guide to Processes, Identification & Preservation. London, Los Angeles: Thames & Hudson, p. 35.
- Source: Pénichon, Sylvie (2013): Twentieth Century Colour Photographs. The Complete Guide to Processes, Identification & Preservation. London, Los Angeles: Thames & Hudson, p. 35.
- Source: Pénichon, Sylvie (2013): Twentieth Century Colour Photographs. The Complete Guide to Processes, Identification & Preservation. London, Los Angeles: Thames & Hudson, p. 36.
- Source: Pénichon, Sylvie (2013): Twentieth Century Colour Photographs. The Complete Guide to Processes, Identification & Preservation. London, Los Angeles: Thames & Hudson, p. 74.
- Source: Pénichon, Sylvie (2013): Twentieth Century Colour Photographs. The Complete Guide to Processes, Identification & Preservation. London, Los Angeles: Thames & Hudson, p. 74.
7 Images
-
![]() Cyan and magenta combined, third layer, yellow missing. Source: Meister Lucius & Brüning (1905): Pinatypie. Positiv-Verfahren für die Dreifarbenphotographie. Höchst am Main.
Cyan and magenta combined, third layer, yellow missing. Source: Meister Lucius & Brüning (1905): Pinatypie. Positiv-Verfahren für die Dreifarbenphotographie. Höchst am Main.
- Source: Pénichon, Sylvie (2013): Twentieth Century Colour Photographs. The Complete Guide to Processes, Identification & Preservation. London, Los Angeles: Thames & Hudson, p. 235.
- Source: Pénichon, Sylvie (2013): Twentieth Century Colour Photographs. The Complete Guide to Processes, Identification & Preservation. London, Los Angeles: Thames & Hudson, p. 235.
- Source: Pénichon, Sylvie (2013): Twentieth Century Colour Photographs. The Complete Guide to Processes, Identification & Preservation. London, Los Angeles: Thames & Hudson, p. 132.
4 Images
-
![]() Credit: Cinémathèque française, conservatoire des techniques, Paris.
Credit: Cinémathèque française, conservatoire des techniques, Paris.
- Source: Wall, E.J. (1925): The History of Three-color Photography. Boston: American Photographic Pub. Co.
2 Images
-
![]() Casanova (FRA 1927, Alexandre Volkoff). Credit: Cinémathèque française. Photographs of the stencil colored safety print by Barbara Flueckiger.
Casanova (FRA 1927, Alexandre Volkoff). Credit: Cinémathèque française. Photographs of the stencil colored safety print by Barbara Flueckiger.
-
Print no. 1 of Amour d'esclave (FRA 1907, Albert Capellani). Credit: Library of Congress. Photograph of the nitrate film print by Barbara Flueckiger.
Edge mark: Pathé (April 1907-1909), on one edge, PATHÉ FRÈRES and on the other, 14 RUE FAVART PARIS (partially visible). Cf. Ill.PM.4: Brown, Harold (1990): Physical Characteristics of Early Films as Aids to Identification. Brussels: FIAF, on p. 9.
- Credit: Cinémathèque française, conservatoire des techniques, Paris.
- Source: Coe, Brian (1981): The History of Movie Photography. Westfield, N.J.: Eastview Editions.
-
Credit: Geo. Willeman, Nitrate Film Vault Manager, Library of Congress. Film: Amour d'esclave (France 1907).
Edge mark: PATHÉ FRÈRES PARIS (without gap, 1906-1907, partially visible). Cf.: Ill.PM.33: Brown, Harold (1990): Physical Characteristics of Early Films as Aids to Identification. Brussels: FIAF, on p. 9.
View Quote on Page: Edge Codes and Identification
Trade mark in scene: Pathé cockerel (until 1909). Cf.: Ill.TM.5: Brown 1990: on p. 30.
- Tinting in combination with stencil coloring. Credit: Turconi Collection by courtesy of George Eastman House Motion Picture Department Collection. Film: L'Exode (FRA 1910, Louis Feuillade)
- Credit: Turconi Collection by courtesy of George Eastman House Motion Picture Department Collection. Film: L'Exode (FRA 1910, Louis Feuillade)
-
Credit: Geo. Willeman, Nitrate Film Vault Manager, Library of Congress. Film: Le Pied de Mouton (ca. 1910).
Edge mark: Pathé (1909 onward), on one edge, PATHÉ FRÈRES 14 RUE FAVART PARIS and on the other, EXHIBITION INTERDITE EN FRANCE EN SUISSE ET EN BELGIQUE (partially visible). Cf.: Ill.PM.5: Brown, Harold (1990): Physical Characteristics of Early Films as Aids to Identification. Brussels: FIAF, on p. 9.
- Credit: Geo. Willeman, Nitrate Film Vault Manager, Library of Congress. Film: The Golden Beetle (1907).
- Credit: Geo. Willeman, Nitrate Film Vault Manager, Library of Congress. Film: Unknown film (ca. 1919).
- Credit: Cinémathèque française, conservatoire des techniques, Paris.
- Credit: Cineteca di Bologna.
- Source: Eggert, John (1932): Kurzer Überblick über den Stand der Farbenkinematographie. In: Bericht über den VIII. Internationalen Kongress für wissenschaftliche und angewandte Photographie, Dresden 1931, pp. 214-222. Leipzig: J. A. Barth.
- Credit: Turconi Collection by courtesy of George Eastman Museum, Motion Picture Department Collection. Film: Kinder-Karno in Nizza.
- Toplight and backlight, Swiss collector's copy. Source: Didiée, L. (1926): Le Film vierge Pathé. Manuel de développement et de tirage. Paris: Pathé.
- Backlight, Swiss collector's copy. Source: Didiée, L. (1926): Le Film vierge Pathé. Manuel de développement et de tirage. Paris: Pathé.
- Toplight and backlight, Swiss collector's copy. Source: Didiée, L. (1926): Le Film vierge Pathé. Manuel de développement et de tirage. Paris: Pathé.
- Toplight and backlight, Swiss collector's copy. Source: Didiée, L. (1926): Le Film vierge Pathé. Manuel de développement et de tirage. Paris: Pathé.
- Pathéorama. Toplight and backlight, Swiss collector's copy. Source: Didiée, L. (1926): Le Film vierge Pathé. Manuel de développement et de tirage. Paris: Pathé.
- Pathéorama. Backlight, Swiss collector's copy. Source: Didiée, L. (1926): Le Film vierge Pathé. Manuel de développement et de tirage. Paris: Pathé.
- Pathéorama. Credit: Clayton Scoble and Stephen Jennings, Harvard University, Fine Arts Library. Backlight. Source: Didiée, L. (1926): Le Film vierge Pathé. Manuel de développement et de tirage. Paris: Pathé.
- Credit: Clayton Scoble and Stephen Jennings, Harvard University, Fine Arts Library. Backlight. Source: Didiée, L. (1926): Le Film vierge Pathé. Manuel de développement et de tirage. Paris: Pathé.
2193 Images in 73 Galleries
-
![]() Source: Wall, E.J. (1925): The History of Three-color Photography. Boston: American Photographic Pub. Co.
Source: Wall, E.J. (1925): The History of Three-color Photography. Boston: American Photographic Pub. Co.
1 Image
-
![]() Source: Pénichon, Sylvie (2013): Twentieth Century Colour Photographs. The Complete Guide to Processes, Identification & Preservation. London, Los Angeles: Thames & Hudson, p. 161.
Source: Pénichon, Sylvie (2013): Twentieth Century Colour Photographs. The Complete Guide to Processes, Identification & Preservation. London, Los Angeles: Thames & Hudson, p. 161.
1 Image
-
![]() Photomicrograph (50x) of an Autochrome mosaic screen. Credit: Courtesy of George Eastman House, International Museum of Photography and Film.
Photomicrograph (50x) of an Autochrome mosaic screen. Credit: Courtesy of George Eastman House, International Museum of Photography and Film.
- Source: Lavédrine, Bertrand (2009): Photographs of the Past. Process and Preservation. Los Angeles: Getty Publications.
- Source: Coote, Jack H. (1993): The Illustrated History of Colour Photography. Surbiton, Surrey: Fountain Press.
- Source: Coote, Jack H. (1993): The Illustrated History of Colour Photography. Surbiton, Surrey: Fountain Press.
- Source: Holme, Charles (1908): Colour Photography, and Other Recent Developments of the Art of the Camera. London, Paris, New York.: Offices of The Studio.
- Source: Holme, Charles (1908): Colour Photography, and Other Recent Developments of the Art of the Camera. London, Paris, New York.: Offices of The Studio.
- Credit: Klosterarchiv Einsiedeln. http://www.klosterarchiv.ch/earchiv_liste.php?Objektyp_physisch=Glasautochrom&start=1
- Credit: Klosterarchiv Einsiedeln. http://www.klosterarchiv.ch/earchiv_liste.php?Objektyp_physisch=Glasautochrom&start=1
- Credit: Klosterarchiv Einsiedeln. http://www.klosterarchiv.ch/earchiv_liste.php?Objektyp_physisch=Glasautochrom&start=1
- Credit: Klosterarchiv Einsiedeln. http://www.klosterarchiv.ch/earchiv_liste.php?Objektyp_physisch=Glasautochrom&start=1
- Pénichon, Sylvie (2013): Twentieth Century Colour Photographs. The Complete Guide to Processes, Identification & Preservation. London, Los Angeles: Thames & Hudson, p. 70.
- Pénichon, Sylvie (2013): Twentieth Century Colour Photographs. The Complete Guide to Processes, Identification & Preservation. London, Los Angeles: Thames & Hudson, p. 26.
- Pénichon, Sylvie (2013): Twentieth Century Colour Photographs. The Complete Guide to Processes, Identification & Preservation. London, Los Angeles: Thames & Hudson, p. 65.
- Pénichon, Sylvie (2013): Twentieth Century Colour Photographs. The Complete Guide to Processes, Identification & Preservation. London, Los Angeles: Thames & Hudson, p. 27.
- Source: Pénichon, Sylvie (2013): Twentieth Century Colour Photographs. The Complete Guide to Processes, Identification & Preservation. London, Los Angeles: Thames & Hudson, p. 235.
- Source: Pénichon, Sylvie (2013): Twentieth Century Colour Photographs. The Complete Guide to Processes, Identification & Preservation. London, Los Angeles: Thames & Hudson, p. 64.
16 Images
Kinemacolor was an additive process operated with alternating red and green filters that were applied to the shutter in front of the camera and in front of the projector. With at least 32 fps the frame rate was double the minimal frame rate of 16 fps. Time parallax with small differences between the red and green record resulted in color fringes that became visible when objects or scenes were moving.
-
![]() Kinemacolor projector used for David Cleveland's and Brian Pritchard's reconstruction. Credit: Brian Pritchard.
Kinemacolor projector used for David Cleveland's and Brian Pritchard's reconstruction. Credit: Brian Pritchard.
13 Images in 3 Galleries
-
![]() Coote, Jack H. (1993): The Illustrated History of Colour Photography. Surbiton, Surrey: Fountain Press,
Coote, Jack H. (1993): The Illustrated History of Colour Photography. Surbiton, Surrey: Fountain Press,
- Credit: Cinémathèque française, conservatoire des techniques, Paris.
- Source: Ede, François (1994): Jour de fête ou la couleur retrouvée. Cahiers du Cinéma: Paris.
- Pénichon, Sylvie (2013): Twentieth Century Colour Photographs. The Complete Guide to Processes, Identification & Preservation. London, Los Angeles: Thames & Hudson, p. 71.
- Source: Pénichon, Sylvie (2013): Twentieth Century Colour Photographs. The Complete Guide to Processes, Identification & Preservation. London, Los Angeles: Thames & Hudson, p. 73.
- Source: Pénichon, Sylvie (2013): Twentieth Century Colour Photographs. The Complete Guide to Processes, Identification & Preservation. London, Los Angeles: Thames & Hudson, p. 31.
- Source: Pénichon, Sylvie (2013): Twentieth Century Colour Photographs. The Complete Guide to Processes, Identification & Preservation. London, Los Angeles: Thames & Hudson, p. 32.
- Source: Pénichon, Sylvie (2013): Twentieth Century Colour Photographs. The Complete Guide to Processes, Identification & Preservation. London, Los Angeles: Thames & Hudson, p. 33.
- Source: Pénichon, Sylvie (2013): Twentieth Century Colour Photographs. The Complete Guide to Processes, Identification & Preservation. London, Los Angeles: Thames & Hudson, p. 48.
- Source: Pénichon, Sylvie (2013): Twentieth Century Colour Photographs. The Complete Guide to Processes, Identification & Preservation. London, Los Angeles: Thames & Hudson, p. 69.
10 Images
-
![]() Principle of capturing and projecting lenticular film. Credit: Joakim Reuteler and Rudolf Gschwind, Digital Humanities Lab, University of Basel, Switzerland. Illustration by Sarah Steinbacher, Multimedia & E-Learning-Services, University of Zurich.
Principle of capturing and projecting lenticular film. Credit: Joakim Reuteler and Rudolf Gschwind, Digital Humanities Lab, University of Basel, Switzerland. Illustration by Sarah Steinbacher, Multimedia & E-Learning-Services, University of Zurich.
- Credit: Illustration by Sarah Steinbacher, Multimedia & E-Learning-Services, University of Zurich. Source: Ede, François (1994): Jour de fête ou la couleur retrouvée. Cahiers du Cinéma: Paris.
- Principle of capturing and projecting lenticular film. Credit: Joakim Reuteler and Rudolf Gschwind, Digital Humanities Lab, University of Basel, Switzerland. Illustration by Sarah Steinbacher, Multimedia & E-Learning-Services, University of Zurich.
- Source: Heymer, Gerd (1933): Auflösungsvermögen und Farbwiedergabe in der Farbrasterphotographie. In: Veröffentlichungen des wissenschaftlichen Zentral-Laboratoriums der photographischen Abteilung Agfa, 3, 1933, pp. 188-207.
4 Images
-
![]() Virages sur mordançage, Bleu (blue mordant toning), backlight, Swiss collectors'copy. Source: Didiée, L. (1926): Le Film vierge Pathé. Manuel de développement et de tirage. Paris: Pathé. [quote id='7']
Virages sur mordançage, Bleu (blue mordant toning), backlight, Swiss collectors'copy. Source: Didiée, L. (1926): Le Film vierge Pathé. Manuel de développement et de tirage. Paris: Pathé. [quote id='7']
- Photomicrograph, 25x. Credit: Norbert Wey, Institute of Pathology, University of Zurich.
- Photomicrograph, 50x. Credit: Norbert Wey, Institute of Pathology, University of Zurich.
- Photomicrograph, 100x. Credit: Norbert Wey, Institute of Pathology, University of Zurich.
- Photomicrograph, 100x. Credit: Norbert Wey, Institute of Pathology, University of Zurich.
171 Images in 7 Galleries
-
![]() Source: Klein, Adrian Bernhard (Cornwell-Clyne) (1940): Colour Cinematography. Boston: American Photographic Pub. Co.
Source: Klein, Adrian Bernhard (Cornwell-Clyne) (1940): Colour Cinematography. Boston: American Photographic Pub. Co.
3 Images in 1 Gallery
-
![]() Credit: Cinémathèque française, conservatoire des techniques, Paris.
Credit: Cinémathèque française, conservatoire des techniques, Paris.
- Credit: Cinémathèque française, conservatoire des techniques, Paris.